Chemistry Matter and Change Online Textbook presents a captivating exploration into the fascinating realm of chemistry. This comprehensive resource delves into the fundamental concepts of matter, delving into its states, properties, and transformations. It unravels the intricacies of chemical reactions, unveiling the factors that govern their rates and equilibrium.
Moreover, this online textbook illuminates the profound relationship between energy and matter, showcasing the diverse forms of energy involved in chemical processes. It explores the practical applications of chemistry, highlighting its indispensable role in various industries and its ethical and environmental implications.
Through engaging narratives and interactive elements, this online textbook invites learners to embark on an immersive journey into the captivating world of chemistry.
Matter: Chemistry Matter And Change Online Textbook
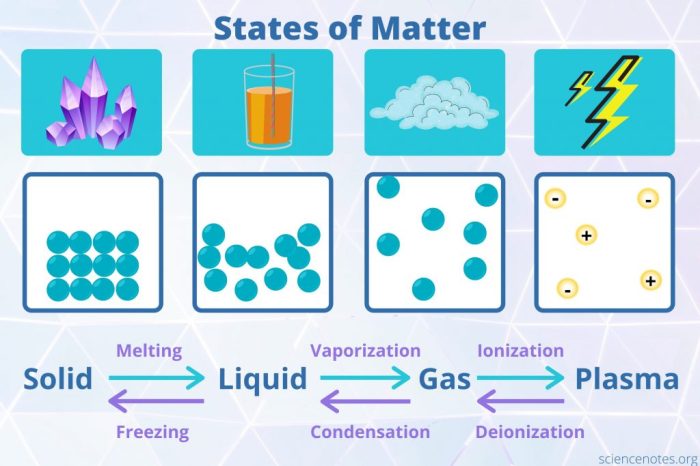
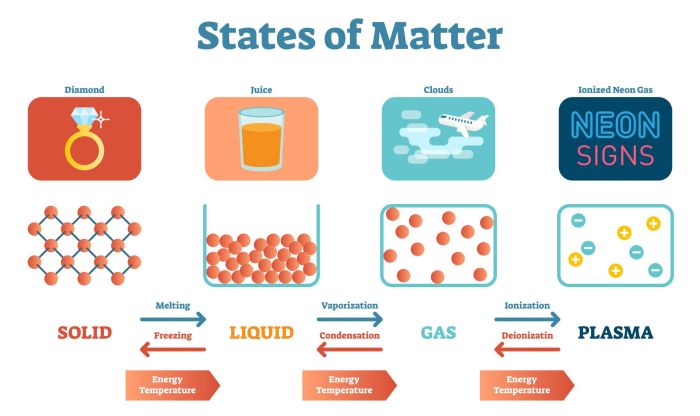
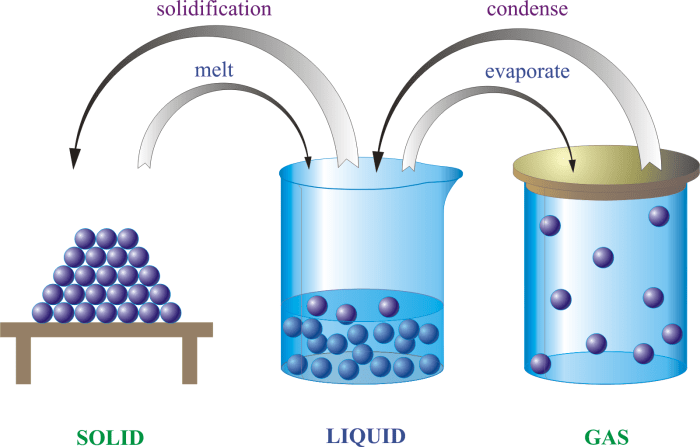
Matter is anything that has mass and takes up space. It can exist in three states: solid, liquid, and gas. Each state has its own unique properties. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have no definite shape or volume.Matter
is made up of atoms, which are the basic building blocks of matter. Atoms are made up of even smaller particles called protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus.
The number of protons in an atom determines what element it is.Matter can be classified into two types: pure substances and mixtures. Pure substances are made up of only one type of atom or molecule, while mixtures are made up of two or more different types of atoms or molecules.
Mixtures can be either homogeneous or heterogeneous. Homogeneous mixtures are mixtures in which the components are evenly distributed throughout the mixture. Heterogeneous mixtures are mixtures in which the components are not evenly distributed throughout the mixture.
Physical and Chemical Changes, Chemistry matter and change online textbook
Matter can undergo two types of changes: physical changes and chemical changes. Physical changes are changes in the form or appearance of matter, but not in its chemical composition. Examples of physical changes include melting, freezing, boiling, and sublimation. Chemical changes are changes in the chemical composition of matter.
Examples of chemical changes include burning, rusting, and cooking.
Chemical Reactions
Chemical reactions are processes in which atoms or molecules are rearranged to form new substances. Chemical reactions can be classified into several types, including synthesis reactions, decomposition reactions, single-replacement reactions, double-replacement reactions, and combustion reactions.The rate of a chemical reaction is the speed at which the reaction occurs.
The rate of a reaction can be affected by several factors, including the concentration of the reactants, the temperature of the reaction, and the presence of a catalyst.Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction are occurring at the same rate.
When a chemical reaction is at equilibrium, the concentrations of the reactants and products do not change over time.
Energy and Matter
Energy and matter are closely related. Energy can be used to create matter, and matter can be used to create energy. The relationship between energy and matter is described by Einstein’s equation E=mc², where E is energy, m is mass, and c is the speed of light.There
are several different forms of energy that can be involved in chemical reactions. These forms of energy include heat, light, and electrical energy. Energy can be used to break bonds between atoms or molecules, or it can be used to form new bonds between atoms or molecules.
Applications of Chemistry
Chemistry is used in a wide variety of applications in everyday life. Chemistry is used in the production of food, clothing, and shelter. It is also used in the development of new technologies, such as medicines and electronic devices.Chemistry is also used in a variety of industries, such as the food industry, the pharmaceutical industry, and the manufacturing industry.
Chemistry is essential for the development of new products and processes that improve our lives.
Tools and Techniques in Chemistry
Chemists use a variety of tools and techniques to study the structure and properties of matter. These tools and techniques include microscopes, spectrometers, and chromatographs.Microscopes are used to magnify objects so that they can be seen in more detail. Spectrometers are used to analyze the composition of matter by measuring the wavelengths of light that it absorbs or emits.
Chromatographs are used to separate mixtures of substances by their different physical properties.Safety is important in the chemistry laboratory. Chemists must wear appropriate safety gear, such as gloves, goggles, and lab coats, when working in the laboratory. They must also follow all safety procedures to avoid accidents.
FAQ Overview
What are the benefits of using Chemistry Matter and Change Online Textbook?
Chemistry Matter and Change Online Textbook offers numerous benefits, including interactive simulations, engaging videos, self-assessment quizzes, and a user-friendly interface that enhances the learning experience and makes chemistry more accessible and enjoyable.
Is Chemistry Matter and Change Online Textbook suitable for all levels of learners?
Yes, Chemistry Matter and Change Online Textbook is designed to cater to a wide range of learners, from beginners to advanced students. Its modular structure and customizable content allow learners to tailor their learning experience to their specific needs and interests.
How can I access Chemistry Matter and Change Online Textbook?
Chemistry Matter and Change Online Textbook is available online through a subscription-based model. Users can purchase individual chapters or subscribe to the entire textbook, providing flexible access to the content.