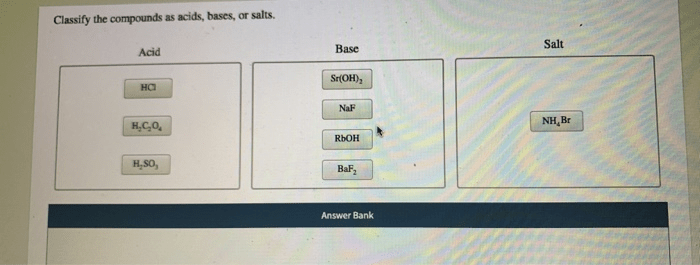
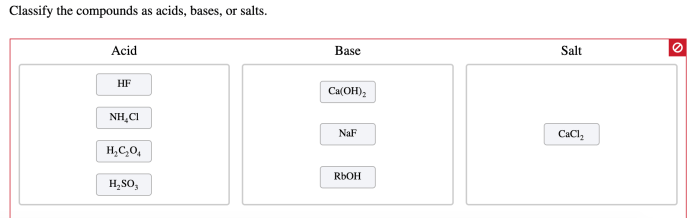
Classify the compounds as acids bases or salts – Classify the compounds as acids, bases, or salts is a crucial task in chemistry, as it provides insights into their properties and reactivity. This comprehensive guide will delve into the characteristics, roles, and classification criteria of acids, bases, and salts, equipping you with a thorough understanding of these fundamental chemical compounds.
Acids, bases, and salts play pivotal roles in various chemical reactions and everyday applications. Understanding their classification is essential for comprehending their behavior and significance in the world around us.
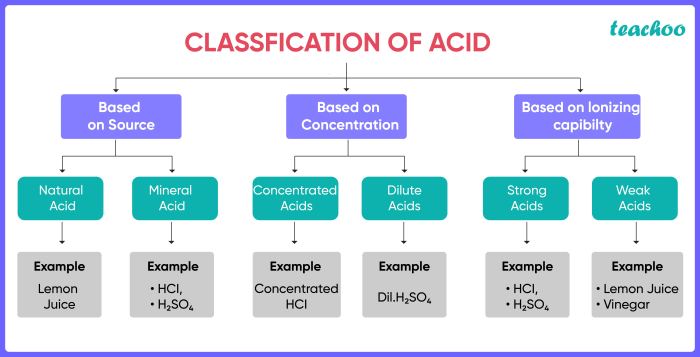
Acids
Acids are chemical compounds that have the following properties:
- They have a sour taste.
- They turn blue litmus paper red.
- They react with metals to produce hydrogen gas.
- They react with bases to produce salts and water.
Common acids include:
- Hydrochloric acid (HCl)
- Sulfuric acid (H2SO4)
- Nitric acid (HNO3)
- Acetic acid (CH3COOH)
Acids play an important role in many chemical reactions, such as:
- Dissolving metals
- Neutralizing bases
- Producing salts
Bases
Bases are chemical compounds that have the following properties:
- They have a bitter taste.
- They turn red litmus paper blue.
- They react with acids to produce salts and water.
Common bases include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)2)
- Ammonia (NH3)
Bases play an important role in many chemical reactions, such as:
- Neutralizing acids
- Producing salts
- Catalysing reactions
Salts
Salts are chemical compounds that are formed when an acid reacts with a base. They have the following properties:
- They are usually solids.
- They are often white or colourless.
- They are soluble in water.
- They do not react with acids or bases.
Common salts include:
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium carbonate (CaCO3)
- Magnesium sulfate (MgSO4)
Salts play an important role in many chemical reactions, such as:
- Providing electrolytes for the body
- Preserving food
- Melting ice
Classification of Compounds: Classify The Compounds As Acids Bases Or Salts
The following table classifies compounds as acids, bases, or salts:
| Compound | Type | Properties | Examples |
|---|---|---|---|
| Hydrochloric acid (HCl) | Acid | Sour taste, turns blue litmus paper red, reacts with metals to produce hydrogen gas, reacts with bases to produce salts and water | Hydrochloric acid, sulfuric acid, nitric acid, acetic acid |
| Sodium hydroxide (NaOH) | Base | Bitter taste, turns red litmus paper blue, reacts with acids to produce salts and water | Sodium hydroxide, potassium hydroxide, calcium hydroxide, ammonia |
| Sodium chloride (NaCl) | Salt | Usually solids, often white or colourless, soluble in water, do not react with acids or bases | Sodium chloride, potassium chloride, calcium carbonate, magnesium sulfate |
Neutralization Reactions
Neutralization reactions are chemical reactions that occur between an acid and a base. The products of a neutralization reaction are a salt and water. Neutralization reactions are important because they can be used to:
- Neutralize the effects of acids or bases
- Produce salts
- Control the pH of a solution
For example, the neutralization reaction between hydrochloric acid and sodium hydroxide produces sodium chloride and water:
Applications of Acids, Bases, and Salts
Acids, bases, and salts have a wide range of applications in everyday life and in various industries. Some examples include:
- Acids are used in the production of fertilizers, dyes, and plastics.
- Bases are used in the production of soaps, detergents, and paper.
- Salts are used in the production of food, fertilizers, and water softeners.
Acids, bases, and salts can also be hazardous if they are not handled properly. It is important to follow the safety precautions when working with these chemicals.
Essential FAQs
What is the pH range of acids?
Acids typically have a pH below 7.
What are some common examples of bases?
Common examples of bases include sodium hydroxide, potassium hydroxide, and ammonia.
How are salts formed?
Salts are formed when an acid reacts with a base in a neutralization reaction.